Category
- Best Peptides for muscle growth
- Geno Pharma Domestic Warehouse 2 (Canada&USA)
- GPH(Domestic Shipping US) Warehouse 1
- Human Pharma Premium
- Phar Labs Premium-Select
- Steroids on Sale USA, Real Steroids Online
- New arrivals in USA
- Most popular steroids in USA
- Antiestrogens / Gonadotropins
- Bangkok Steroid USA
- Biopharma Steroid USA
- British Dragon
- Anabolic Steroids for Horses
- Fat-burners
- Gen Pharma USA
- Medical Pharma Steroid USA
- Medical Tech Steroid USA
- Novocrine Steroids
- HGH USA
- Omega Labs Steroid USA
- Rotterdam Steroids USA
- SARMs USA
- Sciroxx
- Sydgroup Steroid USA
- Big vetenary Steroid USA
- Watson Steroids
- XT Labs Steroids
Most Popular steroids USA
-
 Primobolan for sale 100mg 10 ml Human Pharma in USA
$115.00
Primobolan for sale 100mg 10 ml Human Pharma in USA
$115.00 -
 NPP Steroid 100mg 10 ml Premium Domestic USA
NPP Steroid 100mg 10 ml Premium Domestic USA
$99.00Original price was: $99.00.$80.00Current price is: $80.00. -
 Anavar for Sale in USA – 10mg 80 Tabs GPH-Premium
Anavar for Sale in USA – 10mg 80 Tabs GPH-Premium
$110.00Original price was: $110.00.$80.00Current price is: $80.00. -
 Buy Anavar 10mg – Purchase Geno Pharma
$99.00
Buy Anavar 10mg – Purchase Geno Pharma
$99.00 -
 Testosterone Cypionate Buy 300mg 10ml Geno Pharma
$99.00
Testosterone Cypionate Buy 300mg 10ml Geno Pharma
$99.00 -
 Buy Testosterone E 300mg 10 ml Geno Pharma Domestic USA/CA
$99.00
Buy Testosterone E 300mg 10 ml Geno Pharma Domestic USA/CA
$99.00 -
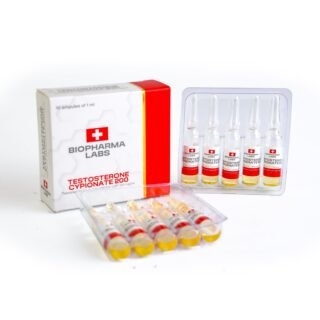
 Testosterone Cypionate 200 Biopharma 10 amp
Testosterone Cypionate 200 Biopharma 10 amp
$99.00Original price was: $99.00.$72.00Current price is: $72.00. -
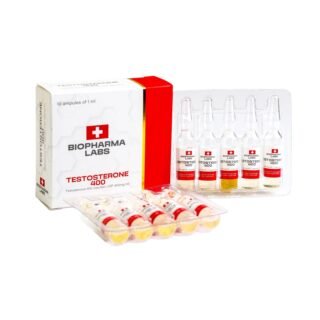
 Testosterone 400 Biopharma 10 Ampoules
Testosterone 400 Biopharma 10 Ampoules
$99.00Original price was: $99.00.$75.00Current price is: $75.00. -
 Eq 300 steroid Rotterdam 10ml
Eq 300 steroid Rotterdam 10ml
$79.00Original price was: $79.00.$69.00Current price is: $69.00. -
 Steroid Deca Geno Pharma 300mg 10ml
Steroid Deca Geno Pharma 300mg 10ml
$110.00Original price was: $110.00.$99.00Current price is: $99.00. -
 Boldenone Cypionate 200 mg / 10 mL Geno Pharma
Boldenone Cypionate 200 mg / 10 mL Geno Pharma
$90.00Original price was: $90.00.$85.00Current price is: $85.00. -
 Somatrox XT Labs 150 IU x 10 vials (15 ui each)
Somatrox XT Labs 150 IU x 10 vials (15 ui each)
$450.00Original price was: $450.00.$350.00Current price is: $350.00.

Androderm Profile
Androderm Background and History
Androderm is a brand name testosterone patch manufactured by Allergan Pharmaceuticals. It was approved by the FDA in 1995 as a treatment for testosterone deficiency in men. Androderm was one of the first transdermal testosterone delivery methods to become available. It provided a convenient way to provide a continuous measured dose of testosterone through the skin.
Androderm gradually lost popularity after testosterone gels became available in the 2000s. However, it remains an FDA approved option for testosterone replacement therapy today. Generic versions are also now available.

Delivery Mechanism
Androderm patches contain a reservoir of testosterone suspended in an alcohol-based gel. When applied to the skin, the testosterone diffuses through the dermis and enters the bloodstream circumventing the liver to avoid rapid metabolism. This provides sustained testosterone levels for 24 hours with just once daily patch application.
Dosing and Administration
Androderm patch dosages range from 2.5mg to 5mg of testosterone per 24 hours. The starting dose is often 2.5mg/day, which can be increased to 5mg/day if needed to restore normal testosterone levels. Patches are applied once every 24 hours onto areas like the back, stomach, upper arms or thighs.
Effects and Benefits
Used properly, Androderm relieves testosterone deficiency symptoms by restoring circulating testosterone levels back to a normal physiological range. This provides benefits such as improved sexual function, increased muscle mass and strength, enhanced energy and mood, and reduced fatigue.
Side Effects and Precautions
Potential side effects may include skin reactions at the application site, acne, male pattern baldness, gynecomastia, testicular atrophy, and adverse lipid profile changes. Androderm should only be used under medical supervision in men clinically diagnosed with low testosterone.
Legal Status
Androderm patches are classified as a Schedule III controlled substance in the United States. A valid prescription is required to legally obtain and use them. Distribution or possession without a prescription violates federal law. Misuse may lead to criminal penalties.
In summary, Androderm transdermal testosterone patches can effectively and conveniently treat clinically diagnosed testosterone deficiency when used properly under medical supervision. Responsible and lawful use is imperative.
Share this page:
- Click to share on X (Opens in new window) X
- Click to share on Facebook (Opens in new window) Facebook
- Click to email a link to a friend (Opens in new window) Email
- Click to share on LinkedIn (Opens in new window) LinkedIn
- Click to share on Reddit (Opens in new window) Reddit
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Telegram (Opens in new window) Telegram
- Click to share on WhatsApp (Opens in new window) WhatsApp
- Click to share on Tumblr (Opens in new window) Tumblr
Pay with WISE APP or Remitly
Pay with WISE App or Remitly
Fast money transfers from USA for fast delivery of steroids
Secure delivery in USA
100% reliable shipping in USA
24x7 Support
Online 24 hours
Low cost delivery
Great shipping prices in USA
BULK ORDER DISCOUNT
If you are a reseller in the USA you can get a special DISCOUNT, we can give you up to 50% or more on bulk orders. If you want to make a bulk order, we can negociate for orders of over USD$4,000, contact us by email.
Steroids info