Category
- Best Peptides for muscle growth
- Geno Pharma Domestic Warehouse 2 (Canada&USA)
- GPH(Domestic Shipping US) Warehouse 1
- Human Pharma Premium
- Phar Labs Premium-Select
- Steroids on Sale USA, Real Steroids Online
- New arrivals in USA
- Most popular steroids in USA
- Antiestrogens / Gonadotropins
- Bangkok Steroid USA
- Biopharma Steroid USA
- British Dragon
- Anabolic Steroids for Horses
- Fat-burners
- Gen Pharma USA
- Medical Pharma Steroid USA
- Medical Tech Steroid USA
- Novocrine Steroids
- HGH USA
- Omega Labs Steroid USA
- Rotterdam Steroids USA
- SARMs USA
- Sciroxx
- Sydgroup Steroid USA
- Big vetenary Steroid USA
- Watson Steroids
- XT Labs Steroids
Most Popular steroids USA
-
 Primobolan for sale 100mg 10 ml Human Pharma in USA
$115.00
Primobolan for sale 100mg 10 ml Human Pharma in USA
$115.00 -
 NPP Steroid 100mg 10 ml Premium Domestic USA
NPP Steroid 100mg 10 ml Premium Domestic USA
$99.00Original price was: $99.00.$80.00Current price is: $80.00. -
 Anavar for Sale in USA – 10mg 80 Tabs GPH-Premium
Anavar for Sale in USA – 10mg 80 Tabs GPH-Premium
$110.00Original price was: $110.00.$80.00Current price is: $80.00. -
 Buy Anavar 10mg – Purchase Geno Pharma
$99.00
Buy Anavar 10mg – Purchase Geno Pharma
$99.00 -
 Testosterone Cypionate Buy 300mg 10ml Geno Pharma
$99.00
Testosterone Cypionate Buy 300mg 10ml Geno Pharma
$99.00 -
 Buy Testosterone E 300mg 10 ml Geno Pharma Domestic USA/CA
$99.00
Buy Testosterone E 300mg 10 ml Geno Pharma Domestic USA/CA
$99.00 -
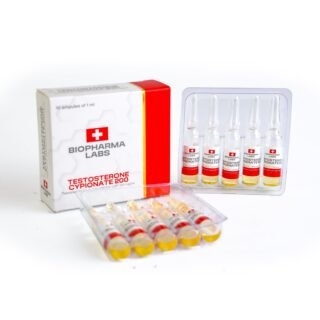
 Testosterone Cypionate 200 Biopharma 10 amp
Testosterone Cypionate 200 Biopharma 10 amp
$99.00Original price was: $99.00.$72.00Current price is: $72.00. -
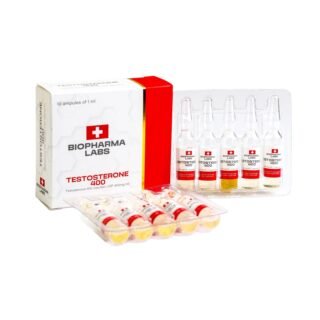
 Testosterone 400 Biopharma 10 Ampoules
Testosterone 400 Biopharma 10 Ampoules
$99.00Original price was: $99.00.$75.00Current price is: $75.00. -
 Eq 300 steroid Rotterdam 10ml
Eq 300 steroid Rotterdam 10ml
$79.00Original price was: $79.00.$69.00Current price is: $69.00. -
 Steroid Deca Geno Pharma 300mg 10ml
Steroid Deca Geno Pharma 300mg 10ml
$110.00Original price was: $110.00.$99.00Current price is: $99.00. -
 Boldenone Cypionate 200 mg / 10 mL Geno Pharma
Boldenone Cypionate 200 mg / 10 mL Geno Pharma
$90.00Original price was: $90.00.$85.00Current price is: $85.00. -
 Somatrox XT Labs 150 IU x 10 vials (15 ui each)
Somatrox XT Labs 150 IU x 10 vials (15 ui each)
$450.00Original price was: $450.00.$350.00Current price is: $350.00.

Metribolone Steroid 1100mcg/ml 10 ml -Buy Metribolone MP
$103.00
Methyltrienolone; METRIBOLONE; 965-93-5; R1881; Metribolona; 17alpha-Methyltrienolone.
C19H24O2
CAS – 965-93-5
A synthetic non-aromatizable androgen and anabolic steroid. It binds strongly to the androgen receptor and has therefore also been used as an affinity label for this receptor in the prostate and in prostatic tumors.
Metribolone contains: 1100mcg.
1100 mcg / ml Methyltrienolone.
Pharmacologic Category: Androgen.
Dosing: Adult 1100 mcg every 24 hours.
Dosing: Geriatric Refer to adult dosing.
Dosing: Pediatric. Has not been tested in pediatric population.
Dosing: Renal Impairment. No dosage adjustment provided in manufacturer’s labeling; use with caution.
Dosing: Hepatic Impairment. No dosage adjustment provided in manufacturer’s labeling; use with caution.
Share this page:
- Share on X (Opens in new window) X
- Share on Facebook (Opens in new window) Facebook
- Email a link to a friend (Opens in new window) Email
- Share on LinkedIn (Opens in new window) LinkedIn
- Share on Reddit (Opens in new window) Reddit
- Share on Pinterest (Opens in new window) Pinterest
- Share on Telegram (Opens in new window) Telegram
- Share on WhatsApp (Opens in new window) WhatsApp
- Share on Tumblr (Opens in new window) Tumblr
Buy Metribolone Steroid – Medical Pharma Anabolic Steroid in USA
Buy Metribolone steroid online in USA SteroidsOnlineUSA.com
Metribolone Steroid use:
Labeled indications
Androgens are primarily indicated in males as replacement therapy when congenital or acquired endogenous androgen absence or deficiency is associated with primary or secondary hypogonadism. Primary hypogonadism includes conditions such as: testicular failure due to cryptorchidism, bilateral torsion, orchitis, or vanishing testis syndrome; orchidectomy, Klinefelter’s syndrome, chemotherapy, or toxic damage from alcohol or heavy metals. Hypogonadotropic hypogonadism (secondary hypogonadism) conditions include idiopathic gonadotropin or LHRH deficiency, or pituitary-hypothalamic injury as a result of tumors, trauma, or radiation and are the most common forms of hypogonadism seen in older adults.
Storage/Stability
Store at room temperature. Protect from light.
Contraindications from Metribolone steroid
Hypersensitivity to Methyltrienolone or any component of the formulation; males with carcinoma of the breast or known or suspected carcinoma of the prostate; women who are breast-feeding, pregnant, or who may become pregnant.
Pregnancy Risk Factor X
Category X: Studies in animals or human beings have demonstrated fetal abnormalities, or there is evidence of fetal risk based on human experience, or both, and the risk of the use of the drug in pregnant women clearly outweighs any possible benefit. The drug is contraindicated in women who are or may become pregnant.
Pregnancy considerations
Methyltrienolone may cause adverse effects, including masculinization of the female fetus, if used during pregnancy. Females who are or may become pregnant should also avoid skin-to-skin contact to areas where testosterone has been applied topically on another person.
Lactation
Contraindicated Breast-Feeding Considerations.
High levels of endogenous maternal testosterone, such as those caused by certain ovarian cysts, suppress milk production. Maternal serum testosterone levels typically decrease after pregnancy and revert to normal upon discontinuing breast-feeding. We do not know the quantity of testosterone in breast milk or its impact on nursing infants after maternal supplementation. Some products should not be used during breast-feeding. Nursing females should refrain from skin-to-skin contact with areas where another person has topically applied testosterone.
Adverse reactions from Metribolone steroid
This compound is highly hepatotoxic.
Cardiovascular
Hypertension (≥3%), increased blood pressure (1%), decreased blood pressure, deep vein thrombosis, edema, vasodilatation.
Central nervous system
Headache (1% to ≥3%), fatigue (2%), irritability (2%), insomnia (≤2%), mood swings (≤2%), aggressive behavior (1%), taste disorder (1%), altered sense of smell (≤1%), abnormal dreams, amnesia, anxiety, chills, depression, dizziness, emotional lability, excitement, hostility, malaise, nervousness, outbursts of anger, paresthesia, seizure, sleep apnea, suicidal ideation
Dermatologic
Acne vulgaris (5%), hyperhidrosis (1%), alopecia, contact dermatitis, diaphoresis, erythema, folliculitis, hair discoloration, pruritus, seborrhea, skin rash, xeroderma.
Endocrine & metabolic
Increased plasma estradiol concentration (3%), weight gain (1%), gynecomastia (≤1%), hot flash (≤1%), change in libido, decreased gonadotropin, fluid retention, hirsutism (increase in pubic hair growth), hypercalcemia, hyperchloremia, hypercholesterolemia, hyperglycemia, hyperkalemia, hyperlipidemia, hypernatremia, hypoglycemia, hypokalemia, inorganic phosphate retention, menstrual disease (including amenorrhea).
Gastrointestinal
Diarrhea (≥3%), gastroesophageal reflux disease, gastrointestinal hemorrhage, gastrointestinal irritation, increased appetite, nausea, vomiting.
Following buccal administration (most common): Dysgeusia, gingival pain, gingival swelling, mouth irritation (including gums), unpleasant taste.
Genitourinary
Prostate specific antigen increase (5% to 11%), prostatitis (≥3%), ejaculatory disorder (1%), prostate induration (1%), spontaneous erections (≤1%), benign prostatic hypertrophy, difficulty in micturition, hematuria, impotence, irritable bladder, mastalgia, oligospermia, priapism, testicular atrophy, urinary tract infection, virilization.
Hepatic
Abnormal hepatic function tests, cholestatic hepatitis, cholestatic jaundice, hepatic insufficiency, hepatic necrosis hepatocellular neoplasms, increased serum bilirubin, peliosis hepatis.
Hematologic & oncologic
Increased hematocrit (1% to 3%), increased hemoglobin (2%), malignant neoplasm of prostate (1%), anemia, clotting factors suppression, hemorrhage, leukopenia, polycythemia, prostate carcinoma.
Hypersensitivity
Anaphylactoid reaction, hypersensitivity reaction (including pulmonary oil microembolism).
Local
Pain at injection site (5%), erythema at injection site (1%), application site reaction (gel, solution), inflammation at injection site.
Transdermal system
Application site pruritus (17% to 37%), application site vesicles (including burn-like blisters under system; 6% to 12%), application site erythema (≤7%), local allergic contact dermatitis (4%), application site burning (3%), application site induration (3%), local skin exfoliation (<3%).
Neuromuscular & skeletal
Arthralgia (≥3%), back pain (≥3%), abnormal bone growth (accelerated), hemarthrosis, hyperkinesia, weakness.
Ophthalmic
Increased lacrimation.
Renal
Increased serum creatinine, polyuria.
Respiratory
Bronchitis (≥3%), nasopharyngitis (≥3%), sinusitis (≥3%), upper respiratory tract infection (≥3%), dyspnea
Drug interactions
Blood Glucose Lowering Agents: Androgens may enhance the hypoglycemic effect of Blood Glucose Lowering Agents. Risk C: Monitor therapy.
C1 inhibitors: Androgens may enhance the thrombogenic effect of C1 inhibitors. Risk C: Monitor therapy.
Corticosteroids (Systemic): May enhance the fluid-retaining effect of Androgens. Risk C: Monitor therapy.
Cyclosporine (Systemic): Androgens may enhance the hepatotoxic effect of Cyclosporine (Systemic). Androgens may increase the serum concentration of Cyclosporine (Systemic). Risk D: Consider therapy modification.
Dehydroepiandrosterone: May enhance the adverse/toxic effect of Testosterone. Risk X: Avoid combination.
Vitamin K Antagonists (eg, warfarin): Androgens may enhance the anticoagulant effect of Vitamin K Antagonists. Risk D: Consider therapy modification.
Test interactions
Methyltrienolone may decrease thyroxine-binding globulin, resulting in decreased total T4; free thyroid hormone levels are not changed.
Monitoring parameters
Liver function tests, cholesterol profile, hemoglobin/hematocrit; INR/PT in patients on anticoagulant therapy.
Children: Radiographs of left wrist and hand every 6 months (to assess bone maturation).
Adult females: Signs of virilization (deepening voice, hirsutism, acne, clitoromegaly); urine and serum calcium in women with breast cancer.
Dosage forms
Oral Tablets, 500mcg.
Injectable, 1100mcg.
Anatomic Therapeutic Chemical (ATC) Classification Not available.
Mechanism of action
Anabolic steroid derived from Trenbolone, sharing some properties. Characterized by its androgenic and anti-glucocorticoid activity. They have modified the molecule of trenbolone to make it orally active by 17-alpha-alkylating it.
Pharmacodynamics/Kinetics from Metribolone steroid
Well absorbed after ingestion with an empty stomach.
Active life: 4-6 hours.
Metabolism: Hepatic.
Buy anabolic steroids online USA.
Related products
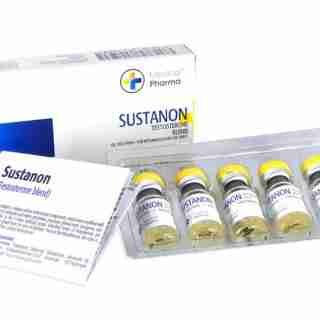
Sustanon 250mg/ml 10ml – Medical Pharma in USA
$95.00Add to cartSustanon contains a mixture of four testosterone compounds, which were modified with the addition of carboxylic acid esters (propionic, propionic phenyl ester, isocaproic, and decanoic acids) at the 17-beta hydroxyl group.
Esterified forms of Testosterone are absorbed more slowly from the area of injection. Once in the bloodstream, the ester is removed to yield free (active) Testosterone.
Mastabol 100mg/ml 10ml – Medical Pharma Buy in USA
$93.00Add to cartMastabol 100mg/ml (10ml) by Medical Pharma is a premium Drostanolone Propionate formula designed to enhance muscle hardness, strength, and fat loss. Ideal for cutting cycles, it promotes a lean, defined physique while maintaining high performance. Available for secure purchase in the USA with fast shipping and a trusted buying process.
Testesterone P 100mg/ml 10ml (Testosterone Propionate) – Medical Pharma
$93.00Add to cartC22H32O3 = 344.5.
CAS — 57-85-2.
ATC — G03BA03.
ATC Vet — QG03BA03.SP 31(Testosterone Propionate). White or creamy-white, odourless, crystals or crystalline powder. Insoluble in water; freely soluble in alcohol, in dioxan, in ether, and in other organic solvents; soluble in vegetable oils. Protect from light.
Testosterone contains: 100mg 100 mg / ml Testosterone Propionate.
Megabol 200mg/ml 10ml – Medical Pharma
$99.00Add to cartMetandienone (pINN); Metandienon; Metandienona; Métandiénone; Metandienoni; Metandienonum; Methandrostenolone; NSC-42722.17β-Hydroxy-17α-methylandrosta-1,4 dien-3-one.
C20H28O2 = 300.4.
CAS — 72-63-9.
ATC — A14AA03; D11AE01.
ATC Vet — QA14AA03; QD11AE01.NSC-9166; Propionato de testosterona; Testosteron Propiyonat; Testostérone, propionate de; Testosteroni propionas; Testosteronipropionaatti; Testosteronopropionatas; Testosteronpropionat; Testosteron-propionát; Testosteronu propionian; Tesztoszteronpropionát. 3-Oxoandrost-4-en-17β-yl propionate; 17β-Hydroxyandrost-4-en-3-one propionate.
Pay with WISE APP or Remitly
Pay with WISE App or Remitly
Fast money transfers from USA for fast delivery of steroids
Secure delivery in USA
100% reliable shipping in USA
24x7 Support
Online 24 hours
Low cost delivery
Great shipping prices in USA
BULK ORDER DISCOUNT
If you are a reseller in the USA you can get a special DISCOUNT, we can give you up to 50% or more on bulk orders. If you want to make a bulk order, we can negociate for orders of over USD$4,000, contact us by email.
Steroids info