Category
- Best Peptides for muscle growth
- Human Pharma Premium
- Phar Labs Premium
- Steroids on Sale USA, Real Steroids Online
- New arrivals in USA
- Most popular steroids in USA
- Antiestrogens / Gonadotropins
- Bangkok Steroid USA
- Biopharma Steroid USA
- British Dragon
- Anabolic Steroids for Horses
- Fat-burners
- Gen Pharma USA
- Medical Pharma Steroid USA
- Medical Tech Steroid USA
- Novocrine Steroids
- HGH USA
- Omega Labs Steroid USA
- Rotterdam Steroids USA
- SARMs USA
- Sciroxx Steroid USA
- Sydgroup Steroid USA
- Big vetenary Steroid USA
- Watson Steroid For Sale
- XT Labs Steroids
Most Popular steroids USA
-
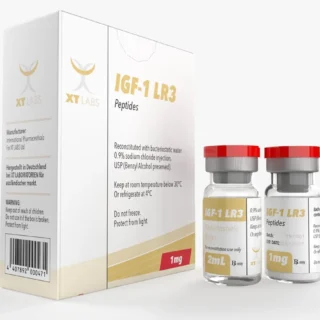
 IGF-1 LR3 1mg 2 ml Xt Labs: Supercharged Muscle Growth
IGF-1 LR3 1mg 2 ml Xt Labs: Supercharged Muscle Growth
$150.00Original price was: $150.00.$130.00Current price is: $130.00. -
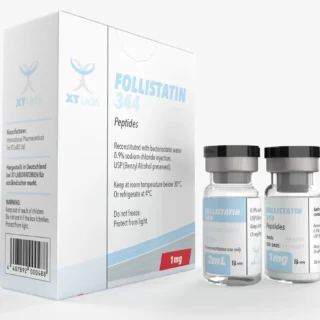
 FOLLISTATIN 344 XT Labs 2 ml 1mg - Benefits, Risks, and Uses
FOLLISTATIN 344 XT Labs 2 ml 1mg - Benefits, Risks, and Uses
$110.00Original price was: $110.00.$90.00Current price is: $90.00. -
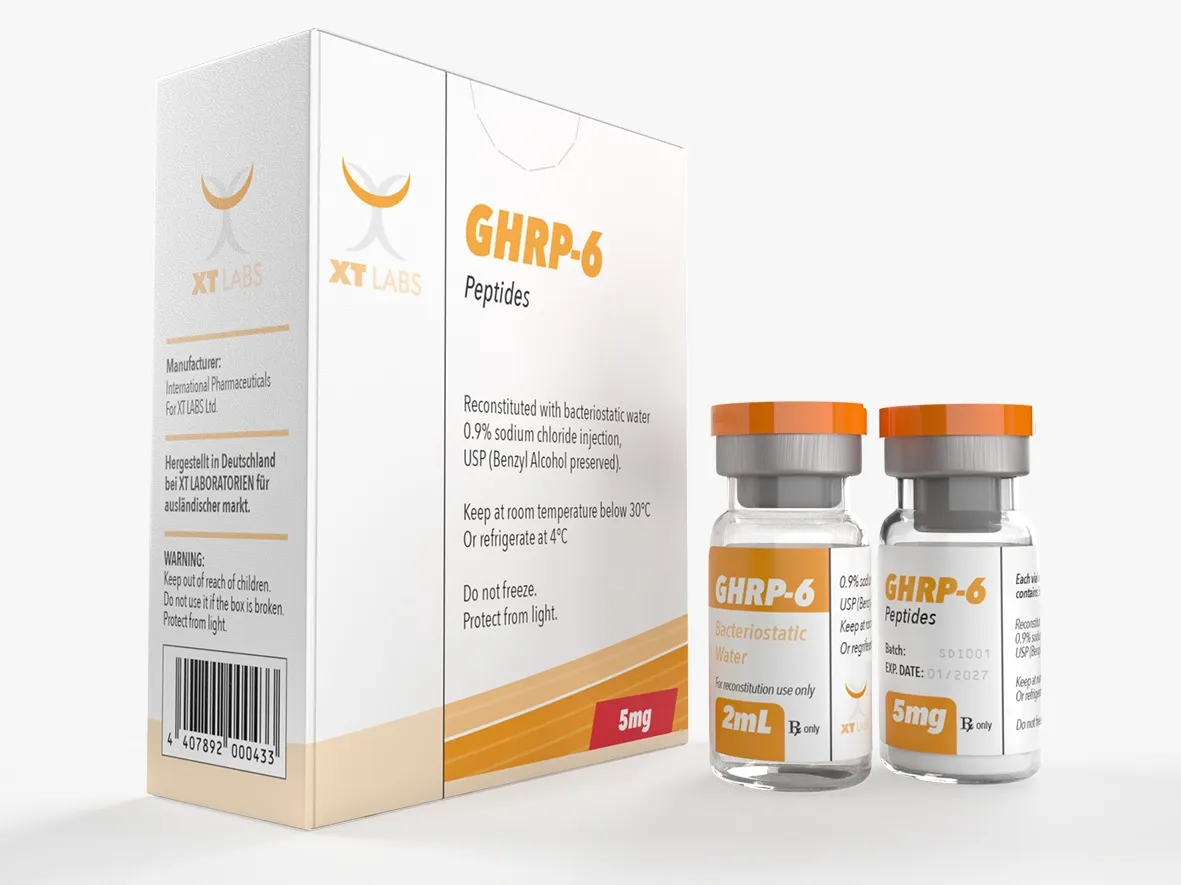
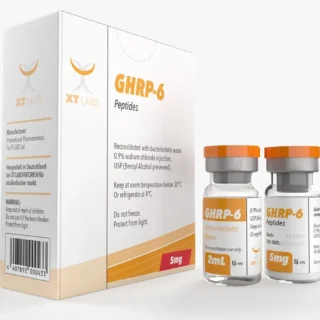
 GHRP 6 Xt Labs 5mg 2 ml - Unlocking Benefits for Fitness
GHRP 6 Xt Labs 5mg 2 ml - Unlocking Benefits for Fitness
$99.00Original price was: $99.00.$79.00Current price is: $79.00.

Testosterone E 250mg/ml 10 ml – Medical Pharma Steroid in USA
$93.00
C26H40O3 = 400.6.
CAS — 315-37-7.
ATC — G03BA03.
ATC Vet — QG03BA03.
USP 31(Testosterone Enanthate). A white or creamy-white crystalline powder. It is odourless or has a faint odour characteristic of heptanoic acid. Insoluble in water; very soluble in ether; soluble in vegetable oils. Store at a temperature of 2°to 8°. Protect from light.
Testosterone E contains: 250mg 250 mg / ml Testosterone Enanthate.
Share this page:
- Click to share on Twitter (Opens in new window)
- Click to share on Facebook (Opens in new window)
- Click to email a link to a friend (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Telegram (Opens in new window)
- Click to share on WhatsApp (Opens in new window)
- Click to share on Tumblr (Opens in new window)
Testosterone E 250mg/ml 10 ml – Medical Pharma Steroid in USA
Testosterone E dosing: Adult
Inoperable metastatic breast cancer (females): IM (testosterone enanthate): 200 to 400 mg every 2 to 4 weeks.
Hypogonadism or hypogonadotropic hypogonadism (males):
IM (testosterone enanthate): 50 to 400 mg every 2 to 4 weeks (FDA-approved dosing range); 75 to 100 mg/week or 150 to 200 mg every 2 weeks.
Delayed puberty (males):
IM (Testosterone enanthate): 50 to 200 mg every 2 to 4 weeks for a limited duration.
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
Delayed puberty (adolescent males):
IM (testosterone enanthate): Refer to adult dosing.
Hypogonadism or hypogonadotropic hypogonadism (adolescent males):
IM (testosterone enanthate): Refer to adult dosing.
Dosing: Renal impairment
There are no dosage adjustments provided in manufacturer’s labeling (has not been studied). Use with caution; may enhance edema formation. Testosterone enanthate is contraindicated in serious renal disease.
Dosing: Hepatic impairmen
There are no dosage adjustments provided in manufacturer’s labeling (has not been studied). Use with caution; may enhance edema formation. Testosterone enanthate is contraindicated in serious hepatic disease.
Use: Labeled indications
Injection: Androgen replacement therapy in the treatment of delayed male puberty; male hypogonadism (primary or hypogonadotropic); inoperable metastatic female breast cancer (enanthate only)
Administration: IM
Administer by deep IM injection into the gluteal muscle.
Testosterone enanthate: Warm to room temperature; shaking vial will help redissolve crystals that have formed after storage.
Hazardous agent; use appropriate precautions for handling and disposal (NIOSH 2014 [group 3]).
Storage/Stability
Store at room temperature. Protect from light.
Preparation for administration
Injection:
Testosterone Enanthate: Warm to room temperature; shaking vial will help redissolve crystals that have formed after storage.
Contraindications
Hypersensitivity to testosterone or any component of the formulation; males with carcinoma of the breast or known or suspected carcinoma of the prostate; women who are breast-feeding, pregnant, or who may become pregnant.
Depo-Testosterone: Also contraindicated in serious hepatic, renal, or cardiac disease.
Documentation of allergenic cross-reactivity for androgens is limited. However, because of similarities in chemical structure and/or pharmacologic actions, the possibility of cross-sensitivity cannot be ruled out with certainty.
Warnings/Precautions
Concerns related to adverse effects:
Cardiovascular events: Studies have suggested an increased risk of cardiovascular events among groups of men prescribed testosterone therapy (Basaria, 2010; Finkle, 2014; Vigen, 2013). The Endocrine Society suggests it may be prudent to avoid testosterone therapy in men who have experienced a cardiovascular event (eg, MI, stroke, acute coronary syndrome) in the past six months (The Endocrine Society, 2014). These risks are currently under review by the FDA (Drug Safety Communication, 2014).
Dyslipidemia: May alter serum lipid profile; use caution with history of MI or coronary artery disease.
Gynecomastia: May cause Gynecomastia.
Hepatic effects: Prolonged use of high doses of oral androgens has been associated with serious hepatic effects (peliosis hepatis, hepatic neoplasms, cholestatic hepatitis, jaundice). Prolonged use of intramuscular testosterone enanthate has been associated with multiple hepatic adenomas. Discontinue therapy if signs or symptoms of hepatic dysfunction (such as jaundice) develop.
Hypercalcemia: May cause hypercalcemia in patients with prolonged immobilization or cancer.
Hypoglycemia: May decrease glucose levels.
Polycythemia: May increase hematocrit requiring dose adjustment or discontinuation. Discontinue therapy if hematocrit exceeds 54%; may reinitiate at lower dose (Bhasin, 2010).
Prostate cancer: May increase the risk of prostate cancer.
Spermatogenesis: Large doses may suppress spermatogenesis.
Venous thromboembolism: Venous thromboembolic events, including deep vein thrombosis (DVT) and pulmonary embolism (PE), have been reported with testosterone products. Evaluate patients with symptoms of pain, edema, warmth, and erythema in the lower extremity for DVT and those with acute shortness of breath for PE. Discontinue therapy if a venous thromboembolism is suspected.
Disease related concerns:
Benign prostatic hyperplasia (BPH): Androgens may worsen BPH; patients may also be at an increased risk of prostate cancer. Discontinue therapy if urethral obstruction develops in patients with BPH (use lower dose if restarted). Withhold therapy pending urological evaluation in patients with palpable prostate nodule or induration, PSA >4 ng/mL, or PSA >3 ng/mL in men at high risk of prostate cancer (Bhasin, 2010).
Diseases exacerbated by fluid retention: Use with caution in patients with diseases that may be exacerbated by fluid retention, including cardiac, hepatic, or renal dysfunction; testosterone may cause fluid retention.
Sleep apnea: May potentiate sleep apnea in some male patients (obesity or chronic lung disease).
Concurrent drug therapy issues:
Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
Elderly: May be inappropriate in this age group due to potential risk of cardiac problems and contraindication for use in men with prostate cancer; in general, avoid use in older adults except in the setting of moderate-to-severe hypogonadism (Beers Criteria). In addition, elderly patients may be at greater risk for prostatic hyperplasia, prostate cancer, fluid retention, and transaminase elevations.
Pediatric: May accelerate bone maturation without producing compensatory gain in linear growth in children; in prepubertal children perform radiographic examination of the hand and wrist every 6 months to determine the rate of bone maturation and to assess the effect of treatment on the epiphyseal centers. Gels, solution, transdermal, and buccal system have not been evaluated in males <18 years of age; safety and efficacy of injection have not been established in males <12 years of age.
Women: During treatment for metastatic breast cancer, women should be monitored for signs of virilization; discontinue if mild virilization is present to prevent irreversible symptoms.
Dosage form specific issues:
Benzyl alcohol and derivatives: Some dosage forms may contain benzyl alcohol; large amounts of benzyl alcohol (≥99 mg/kg/day) have been associated with a potentially fatal toxicity (“gasping syndrome”) in neonates; the “gasping syndrome” consists of metabolic acidosis, respiratory distress, gasping respirations, CNS dysfunction (including convulsions, intracranial hemorrhage), hypotension, and cardiovascular collapse (AAP, 1997; CDC, 1982); some data suggests that benzoate displaces bilirubin from protein binding sites (Ahlfors, 2001); avoid or use dosage forms containing benzyl alcohol with caution in neonates. See manufacturer’s labeling.
Castor oil: Some products may contain castor oil.
Soy: Some testosterone products may be chemically synthesized from soy.
Special handling:
Hazardous agent: Use appropriate precautions for handling and disposal (NIOSH 2014 [group 3]).
Other warnings/precautions:
Abuse/misuse/diversion: Anabolic steroids may be abused; abuse may be associated with adverse physical and psychological effects.
Geriatric considerations
Elderly males treated with androgens may be at increased risk of developing prostatic hyperplasia and prostatic carcinoma. Increase in libido may occur.
Pregnancy risk factor
X (Contraindicated)
Category X: Studies in animals or human beings have demonstrated fetal abnormalities, or there is evidence of fetal risk based on human experience, or both, and the risk of the use of the drug in pregnant women clearly outweighs any possible benefit. The drug is contraindicated in women who are or may become pregnant.
Pregnancy considerations
Testosterone may cause adverse effects, including masculinization of the female fetus, if used during pregnancy.
Lactation
Enters breast milk – contraindicated in lactation
Breast feeding considerations
High levels of endogenous maternal testosterone, such as those caused by certain ovarian cysts, suppress milk production. Maternal serum testosterone levels generally fall following pregnancy and return to normal once breast-feeding is stopped. The amount of testosterone present in breast milk or the effect to the nursing infant following maternal supplementation is not known. Some products are contraindicated while breast-feeding.
Adverse reactions
Frequency not always defined.
Cardiovascular: Hypertension (≥3%), increased blood pressure (1%), decreased blood pressure, deep vein thrombosis, edema, vasodilatation.
Central nervous system: Headache (1% to ≥3%), fatigue (2%), irritability (2%), insomnia (≤2%), mood swings (≤2%), aggressive behavior (1%), taste disorder (1%), altered sense of smell (≤1%), abnormal dreams, amnesia, anxiety, chills, depression, dizziness, emotional lability, excitement, hostility, malaise, nervousness, outbursts of anger, paresthesia, seizure, sleep apnea, suicidal ideation
Dermatologic: Acne vulgaris (5%), hyperhidrosis (1%), alopecia, contact dermatitis, diaphoresis, erythema, folliculitis, hair discoloration, pruritus, seborrhea, skin rash, xeroderma.
Endocrine & metabolic: Increased plasma estradiol concentration (3%), weight gain (1%), gynecomastia (≤1%), hot flash (≤1%), change in libido, decreased gonadotropin, fluid retention, hirsutism (increase in pubic hair growth), hypercalcemia, hyperchloremia, hypercholesterolemia, hyperglycemia, hyperkalemia, hyperlipidemia, hypernatremia, hypoglycemia, hypokalemia, inorganic phosphate retention, menstrual disease (including amenorrhea).
Gastrointestinal: Diarrhea (≥3%), gastroesophageal reflux disease, gastrointestinal hemorrhage, gastrointestinal irritation, increased appetite, nausea, vomiting.
Genitourinary: Prostate specific antigen increase (5% to 11%), prostatitis (≥3%), ejaculatory disorder (1%), prostate induration (1%), spontaneous erections (≤1%), benign prostatic hypertrophy, difficulty in micturition, hematuria, impotence, irritable bladder, mastalgia, oligospermia, priapism, testicular atrophy, urinary tract infection, virilization.
Hepatic: Abnormal hepatic function tests, cholestatic hepatitis, cholestatic jaundice, hepatic insufficiency, hepatic necrosis, hepatocellular neoplasms, increased serum bilirubin, peliosis hepatis.
Hematologic & oncologic: Increased hematocrit (1% to 3%), increased hemoglobin (2%), malignant neoplasm of prostate (1%), anemia, clotting factors suppression, hemorrhage, leukopenia, polycythemia, prostate carcinoma.
Hypersensitivity: Anaphylactoid reaction, hypersensitivity reaction (including pulmonary oil microembolism).
Local: Pain at injection site (5%), erythema at injection site (1%), application site reaction (gel, solution), inflammation at injection site
Neuromuscular & skeletal: Arthralgia (≥3%), back pain (≥3%), abnormal bone growth (accelerated), hemarthrosis, hyperkinesia, weakness.
Ophthalmic: Increased lacrimation
Renal: Increased serum creatinine, polyuria.
Respiratory: Bronchitis (≥3%), nasopharyngitis (≥3%), sinusitis (≥3%), upper respiratory tract infection (≥3%), dyspnea
<1%, postmarketing, and/or case reports: Injection, gel: Abdominal pain, abnormal erythropoiesis, abscess at injection site, allergic dermatitis, anaphylactic shock, anaphylaxis, androgenetic alopecia, angina pectoris, angioedema, asthma, breast induration, cardiac arrest, cardiac failure, cerebral infarction, cerebrovascular accident, cerebrovascular insufficiency, chest pain, chronic obstructive pulmonary disease, circulatory shock, cognitive dysfunction, confusion, coronary artery disease, coronary occlusion, cough, decreased plasma testosterone, decreased thyroxine binding globulin, decreased urinary calcium excretion, diabetes mellitus, dysuria, electrolyte disturbance, epididymitis, erectile dysfunction, hearing loss (sudden), hematoma at injection site, hepatotoxicity (idiosyncratic) (Chalasani, 2014), hyperparathyroidism, hypersensitivity angiitis, hypertriglyceridemia, hyperventilation, impaired glucose tolerance, increased gamma-glutamyl transferase, increased intraocular pressure, increased serum ALT, increased serum AST, increased serum prolactin, increased serum transaminases, increased serum triglycerides, Korsakoff’s psychosis (nonalcoholic), migraine, musculoskeletal chest pain, musculoskeletal pain, myalgia, myocardial infarction, nephrolithiasis, nipple tenderness, orgasm disturbance (male), osteopenia, osteoporosis, peripheral edema, personality disorder, pharyngeal edema, pharyngolaryngeal pain, prolonged partial thromboplastin time, prolonged prothrombin time, prostatic intraepithelial neoplasia, pulmonary embolism, pure red cell aplasia, renal colic, renal pain, respiratory distress, restlessness, reversible ischemic neurological deficit, rhinitis, sleep disorder, snoring, spermatocele, syncope, systemic lupus erythematosus, tachycardia, testicular pain, thrombocytopenia, thromboembolism, thrombosis, tinnitus, transient ischemic attacks, urinary incontinence, urolithiasis, urticaria, venous insufficiency, venous thromboembolism, vesicobullous rash, virilization (of children, following secondary exposure to topical gel [advanced bone age, aggressive behavior, enlargement of clitoris requiring surgery, enlargement of penis, increased erections, increased libido, pubic hair development]), vitreous detachment, voice disorder.
Metabolism/Transport effects
Drug Interactions
Blood Glucose Lowering Agents: Androgens may enhance the hypoglycemic effect of Blood Glucose Lowering Agents. Risk C: Monitor therapy.
C1 inhibitors: Androgens may enhance the thrombogenic effect of C1 inhibitors. Risk C: Monitor therapy.
Corticosteroids (Systemic): May enhance the fluid-retaining effect of Androgens. Risk C: Monitor therapy.
Cyclosporine (Systemic): Androgens may enhance the hepatotoxic effect of Cyclosporine (Systemic). Androgens may increase the serum concentration of Cyclosporine (Systemic). Risk D: Consider therapy modification.
Dehydroepiandrosterone: May enhance the adverse/toxic effect of Testosterone. Risk X: Avoid combination
Vitamin K Antagonists (eg, warfarin): Androgens may enhance the anticoagulant effect of Vitamin K Antagonists. Risk D: Consider therapy modification.
Test interactions
Testosterone may decrease thyroxine-binding globulin, resulting in decreased total T4; free thyroid hormone levels are not changed.
Monitoring parameters
Periodic liver function tests, lipid panel, hemoglobin and hematocrit (prior to therapy, at 3 to 6 months, then annually); radiologic examination of wrist and hand every 6 months (when using in prepubertal children). Withhold initial treatment with hematocrit >50% (discontinue therapy if hematocrit exceeds 54% [Bhasin, 2010]), hyperviscosity, untreated obstructive sleep apnea, or uncontrolled severe heart failure. Monitor urine and serum calcium and signs of virilization in women treated for breast cancer. Serum glucose (may be decreased by testosterone, monitor patients with diabetes). Evaluate males for response to treatment and adverse events 3 to 6 months after initiation and then annually.
Bone mineral density: Monitor after 1 to 2 years of therapy in hypogonadal men with osteoporosis or low trauma fracture (Bhasin, 2010)
PSA: In men >40 years of age with baseline PSA >0.6 ng/mL, PSA and prostate exam (prior to therapy, at 3 to 6 months, then as based on current guidelines). Withhold treatment pending urological evaluation in patients with palpable prostate nodule or induration or PSA >4 ng/mL or if PSA >3 ng/mL in men at high risk of prostate cancer (Bhasin, 2010).
Do not treat with severe untreated BPH with IPSS symptom score >19.
Serum testosterone: After initial dose titration (if applicable), monitor 3 to 6 months after initiating treatment, then annually.
Injection:
Testosterone enanthate: Measure midway between injections. Adjust dose or frequency if testosterone concentration is <400 ng/dL or >700 ng/dL (Bhasin, 2010).
Testosterone undecanoate: Measure just prior to each subsequent injection and adjust dosing interval to maintain serum testosterone in mid-normal range (Bhasin, 2010).
Reference Range
Total testosterone, males:
12 to 13 years: <800 ng/dL
14 years: <1200 ng/dL
15 to 16 years: 100 to 1200 ng/dL
17 to 18 years: 300 to 1200 ng/dL
19 to 40 years: 300 to 950 ng/dL
>40 years: 240 to 950 ng/dL
Free testosterone, males: 9 to 30 ng/dL
Dosage forms
Solution, Intramuscular, as enanthate:
Generic: 200 mg/mL (5 mL)
Anatomic Therapeutic Chemical (ATC) Classification
G03BA03
Mechanism of action
Principal endogenous androgen responsible for promoting the growth and development of the male sex organs and maintaining secondary sex characteristics in androgen-deficient males.
Pharmacodynamics/Kinetics
Duration (route and ester dependent): IM: Cypionate and enanthate esters have longest duration, ≤2 to 4 weeks; Undecanoate 10 weeks; Gel: 24 to 48 hours; buccal system: 2 to 4 hours after removal.
Protein binding: 98%; bound to sex hormone-binding globulin (40%) and albumin.
Metabolism: Hepatic; forms metabolites, including dihydrotestosterone (DHT) and estradiol (both active).
Half-life elimination: Variable: 10 to 100 minutes.
Excretion: Urine (90%); feces (6%).
Effects on bleeding
No information available to require special precautions.
Buy steroids online USA.
Related products
Provir (Mesterolone) 50mg/tab 100 tabs – Medical Pharma Steroid in USA
$93.00Add to cartPharmacologic Category
Androgen.
Dosing: Adult
50mg every days for duration of 4-8 weeks.
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
No information in pediatric population available.
Dosing: Renal impairment
No dosage adjustment provided in manufacturer’s labeling; use with caution due to propensity to cause edema.
Dosing: Hepatic impairment
No dosage adjustment provided in manufacturer’s labeling; use with caution.
Anavar For Sale 1# Best USA
$99.00Add to cartAnavar (Oxandrolone) 15mg/tab 100 tabs – Medical Pharma
NSC-67068; Oxandrolona; Oxandrolonum; SC-11585. 17β-Hydroxy-17α-methyl-2-oxa-5α-androstan-3-one.Deposteron Testosterone Cypionate for sale 250mg/ml 10 ml M P Buy in USA
$93.00Add to cartDeposteron
C27H40O3 = 412.6.
CAS — 58-20-8.
ATC — G03BA03.
ATC Vet — QG03BA03.USP 31(Testosterone Cypionate). A white or creamy-white, crystalline powder, odourless or has a slight odour. Insoluble in water; freely soluble in alcohol, in chloroform, in dioxan, and in ether; soluble in vegetable oils. Protect from light.
Testerone C contains: 250mg.
⦁ 250mg/ml Testosterone Cypionate.
Testesterone P 100mg/ml 10ml (Testosterone Propionate) – Medical Pharma Steroid in USA
$93.00Add to cartC22H32O3 = 344.5.
CAS — 57-85-2.
ATC — G03BA03.
ATC Vet — QG03BA03.SP 31(Testosterone Propionate). White or creamy-white, odourless, crystals or crystalline powder. Insoluble in water; freely soluble in alcohol, in dioxan, in ether, and in other organic solvents; soluble in vegetable oils. Protect from light.
Testosterone contains: 100mg 100 mg / ml Testosterone Propionate.
Pay with WISE APP or Remitly
Pay with WISE App or Remitly
Fast money transfers from USA for fast delivery of steroids
Secure delivery in USA
100% reliable shipping in USA
24x7 Support
Online 24 hours
Low cost delivery
Great shipping prices in USA
BULK ORDER DISCOUNT
If you are a reseller in the USA you can get a special DISCOUNT, we can give you up to 50% or more on bulk orders. If you want to make a bulk order, we can negociate for orders of over USD$4,000, contact us by email.
Steroids info