Category
- Best Peptides for muscle growth
- Geno Pharma Domestic Warehouse 2 (Canada&USA) (Ships in 1–3 days) Faster!
- GP(Domestic Shipping US) Warehouse 1
- Human Pharma Premium
- Phar Labs Premium-Select
- Steroids on Sale USA, Real Steroids Online
- New arrivals in USA
- Most popular steroids in USA
- Antiestrogens / Gonadotropins
- Bangkok Steroid USA
- Biopharma Steroid USA
- British Dragon
- Anabolic Steroids for Horses
- Fat-burners
- Gen Pharma USA
- Medical Pharma Steroid USA
- Medical Tech Steroid USA
- Novocrine Steroids
- HGH USA
- Omega Labs Steroid USA
- Rotterdam Steroids USA
- SARMs USA
- Sciroxx
- Sydgroup Steroid USA
- Big vetenary Steroid USA
- Watson Steroids
- XT Labs Steroids
Most Popular steroids USA
-
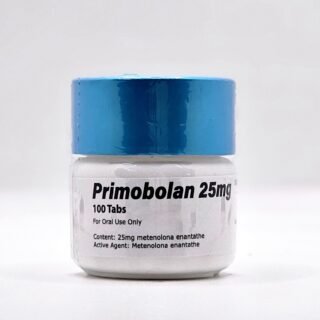
 Primobolan Pills 25mg 100 pills Domestic USA
Primobolan Pills 25mg 100 pills Domestic USA
$99.00Original price was: $99.00.$85.00Current price is: $85.00. -
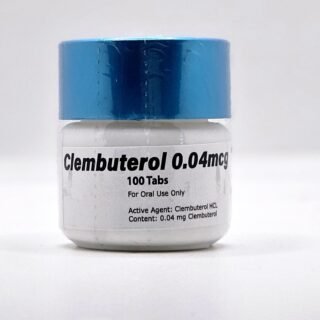
 Clenbuterol for Sale 40mcg 100 Tabs - GP Premium Domestic USA
Clenbuterol for Sale 40mcg 100 Tabs - GP Premium Domestic USA
$99.00Original price was: $99.00.$65.00Current price is: $65.00. -
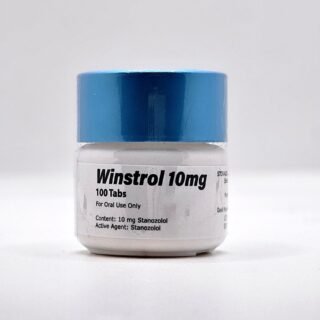
 Winstrol Tablets for Sale 10mg 100 pills GP Domestic
Winstrol Tablets for Sale 10mg 100 pills GP Domestic
$99.00Original price was: $99.00.$75.00Current price is: $75.00.

Deposteron Testosterone Cypionate for sale 250mg/ml 10 ml M P Buy in USA
$93.00
Deposteron
C27H40O3 = 412.6.
CAS — 58-20-8.
ATC — G03BA03.
ATC Vet — QG03BA03.
USP 31(Testosterone Cypionate). A white or creamy-white, crystalline powder, odourless or has a slight odour. Insoluble in water; freely soluble in alcohol, in chloroform, in dioxan, and in ether; soluble in vegetable oils. Protect from light.
Testerone C contains: 250mg.
⦁ 250mg/ml Testosterone Cypionate.
Share this page:
- Click to share on X (Opens in new window) X
- Click to share on Facebook (Opens in new window) Facebook
- Click to email a link to a friend (Opens in new window) Email
- Click to share on LinkedIn (Opens in new window) LinkedIn
- Click to share on Reddit (Opens in new window) Reddit
- Click to share on Pinterest (Opens in new window) Pinterest
- Click to share on Telegram (Opens in new window) Telegram
- Click to share on WhatsApp (Opens in new window) WhatsApp
- Click to share on Tumblr (Opens in new window) Tumblr
Deposteron 250 Testosterone Cypionate 250mg/ml 10 ml (Testosterone Cypionate) – Medical Pharma Steroids in USA
What is Deposteron?
Deposteron is a brand name for a medication that contains testosterone, a hormone that the human body naturally produces. Doctors use it as hormone replacement therapy when men have testosterone deficiency or low testosterone levels. Medical professionals typically administer Deposteron through intramuscular injections, and it helps restore normal testosterone levels. This can bring about various positive effects on a person’s health and well-being, including increased energy levels, muscle mass, and an enhanced mood. It is important to use Deposteron only under the supervision and prescription of a qualified healthcare provider, as improper use can lead to side effects and health risks.
Desposteron Testosterone for sale C dosing: Adult
Hypogonadism or hypogonadotropic hypogonadism (males):
IM: 50 to 400 mg every 2 to 4 weeks (FDA-approved dosing range); 75 to 100 mg/week or 150 to 200 mg every 2 weeks.
Delayed puberty (males):
IM: 50 to 200 mg every 2 to 4 weeks for a limited duration.
Dosing: Geriatric
Refer to adult dosing.
Dosing from Deposteron 250 : Pediatric
Delayed puberty (adolescent males):
IM: Refer to adult dosing.
Hypogonadism or hypogonadotropic hypogonadism (adolescent males):
IM: Refer to adult dosing.
Dosing: Renal impairment
There are no dosage adjustments (has not been studied). Use with caution; may enhance edema formation. Testosterone cypionate is contraindicated in serious renal disease.
Dosing: Hepatic impairment
There are no dosage adjustments (has not been studied). Use with caution; may enhance edema formation. Testosterone cypionate is contraindicated in serious hepatic disease.
Use: Labeled indications from Deposteron
Injection: Androgen replacement therapy in the treatment of delayed male puberty; male hypogonadism (primary or hypogonadotropic); inoperable metastatic female breast cancer (enanthate only).
Administration from Deposteron 250: IM
Deposteron is administered by deep IM injection into the gluteal muscle.
Testosterone enanthate or testosterone cypionate: Warm to room temperature; shaking vial will help redissolve crystals that have formed after storage.
Hazardous agent; use appropriate precautions for handling and disposal (NIOSH 2014 [group 3]).
Storage/Stability
Store at room temperature. Protect from light.
Preparation for administration for Deposteron 250
Injection:
Testosterone enanthate, testosterone cypionate: Warm to room temperature; shaking vial will help redissolve crystals that have formed after storage.
Contraindications with Deposteron 250
Hypersensitivity to testosterone or any component of the formulation; males with carcinoma of the breast or known or suspected carcinoma of the prostate; women who are breast-feeding, pregnant, or who may become pregnant.
Depo-Testosterone: Also contraindicated in serious hepatic, renal, or cardiac disease
Documentation of allergenic cross-reactivity for androgens is limited. However, because of similarities in chemical structure and/or pharmacologic actions, the possibility of cross-sensitivity cannot be ruled out with certainty.
Warnings/Precautions for Deposteron 250
Concerns related to adverse effects:
Cardiovascular events: Studies have suggested an increased risk of cardiovascular events among groups of men prescribed testosterone therapy (Basaria, 2010; Finkle, 2014; Vigen, 2013). The Endocrine Society suggests it may be prudent to avoid testosterone therapy in men who have experienced a cardiovascular event (eg, MI, stroke, acute coronary syndrome) in the past six months (The Endocrine Society, 2014). These risks are currently under review by the FDA (Drug Safety Communication, 2014).
Dyslipidemia: May alter serum lipid profile; use caution with history of MI or coronary artery disease.
Gynecomastia: May cause Gynecomastia.
Hepatic effects: Prolonged use of high doses of oral androgens has linked serious hepatic effects (such as peliosis hepatis, hepatic neoplasms, cholestatic hepatitis, jaundice) to them. Prolonged use of intramuscular testosterone enanthate has linked it to multiple hepatic adenomas. Discontinue therapy if signs or symptoms of hepatic dysfunction (such as jaundice) develop.
Hypercalcemia: May cause hypercalcemia in patients with prolonged immobilization or cancer.
Polycythemia: May increase hematocrit requiring dose adjustment or discontinuation. Discontinue therapy if hematocrit exceeds 54%; may reinitiate at a lower dose (Bhasin, 2010).
Prostate cancer: May increase the risk of prostate cancer.
Spermatogenesis: Large doses may suppress spermatogenesis.
Venous thromboembolism: Venous thromboembolic events, including deep vein thrombosis (DVT) and pulmonary embolism (PE), have been reported with testosterone products. Evaluate patients with symptoms of pain, edema, warmth, and erythema in the lower extremity for DVT and those with acute shortness of breath for PE. Discontinue therapy if a venous thromboembolism is suspected.
Disease-related concerns :
Benign prostatic hyperplasia (BPH): Androgens may worsen BPH; patients may also be at an increased risk of prostate cancer. Discontinue therapy if urethral obstruction develops in patients with BPH (use a lower dose if restarted). Withhold therapy pending urological evaluation in patients with a palpable prostate nodule or induration, PSA >4 ng/mL, or PSA >3 ng/mL in men at high risk of prostate cancer (Bhasin, 2010).
Diseases exacerbated by fluid retention: Use with caution in patients with diseases that may be exacerbated by fluid retention, including cardiac, hepatic, or renal dysfunction; testosterone may cause fluid retention.
Sleep apnea: May potentiate sleep apnea in some male patients (obesity or chronic lung disease).
Concurrent drug therapy issues:
Drug-drug interactions: Potentially significant interactions may exist, requiring dose or frequency adjustment, additional monitoring, and/or selection of alternative therapy. Consult drug interactions database for more detailed information.
Special populations:
In the elderly population, caution should be exercised when considering this medication due to the potential risk of cardiac problems and contraindications for men with prostate cancer. In general, it is advisable to avoid its use in older adults, except in cases of moderate-to-severe hypogonadism as per the Beers Criteria. Furthermore, elderly patients may face an increased risk of prostatic hyperplasia, prostate cancer, fluid retention, and transaminase elevations.
Pediatric: May accelerate bone maturation without producing compensatory gain in linear growth in children; in prepubertal children perform radiographic examination of the hand and wrist every 6 months to determine the rate of bone maturation and to assess the effect of treatment on the epiphyseal centers. Gels, solution, transdermal, and buccal systems have not been evaluated in males <18 years of age; safety and efficacy of injection have not been established in males <12 years of age.
Women: During treatment for metastatic breast cancer, women should be monitored for signs of virilization; discontinue if mild virilization is present to prevent irreversible symptoms.
Dosage from Deposteron 250 for specific issues:
Benzyl alcohol and derivatives: Some dosage forms may contain benzyl alcohol; we have associated large amounts of benzyl alcohol (≥99 mg/kg/day) with potentially fatal toxicity in neonates, known as “gasping syndrome.” This syndrome comprises metabolic acidosis, respiratory distress, gasping respirations, CNS dysfunction (including convulsions, intracranial hemorrhage), hypotension, and cardiovascular collapse (AAP, 1997; CDC, 1982); some data suggests that benzoate displaces bilirubin from protein binding sites (Ahlfors, 2001); avoid or use dosage forms containing benzyl alcohol with caution in neonates. See manufacturer’s labeling.
⦁ Castor oil: Some products may contain castor oil.
⦁ Soy: Some testosterone products may be chemically synthesized from soy.
Special handling from Deposteron:
⦁ Hazardous agent: Use appropriate precautions for handling and disposal (NIOSH 2014 [group 3]).
Other warnings/precautions:
⦁ Abuse/misuse/diversion: Anabolic steroids may be abused; abuse may be associated with adverse physical and psychological effects.
Geriatric considerations with Deposteron 250
Elderly males treated with androgens may be at increased risk of developing prostatic hyperplasia and prostatic carcinoma. Increase in libido may occur.
Pregnancy risk factor with Deposteron use
X (Contraindicated)
Category X: Studies in animals or human beings have demonstrated fetal abnormalities, or there is evidence of fetal risk based on human experience, or both, and the risk of the use of the drug in pregnant women clearly outweighs any possible benefit. The drug is contraindicated in women who are or may become pregnant.
Pregnancy considerations with Deposteron use
Testosterone may cause adverse effects, including masculinization of the female fetus, if used during pregnancy.
Lactation
Enters breast milk – contraindicated in lactation
Breast-Feeding Considerations
Elevated levels of endogenous maternal testosterone, as seen in cases involving certain ovarian cysts, can inhibit milk production. Furthermore, maternal serum testosterone levels generally decrease after pregnancy and return to normal once the mother discontinues breastfeeding. However, the exact quantity of testosterone in breast milk and its impact on the nursing infant following maternal supplementation remain unknown. Consequently, some products should be avoided during breastfeeding due to contraindications
Deposteron Adverse Reactions
Frequency not always defined.
Cardiovascular
Hypertension (≥3%), increased blood pressure (1%), decreased blood pressure, deep vein thrombosis, edema, vasodilatation.
Central Nervous System
Headache (1% to ≥3%), fatigue (2%), irritability (2%), insomnia (≤2%), mood swings (≤2%), aggressive behavior (1%), taste disorder (1%), altered sense of smell (≤1%), abnormal dreams, amnesia, anxiety, chills, depression, dizziness, emotional lability, excitement, hostility, malaise, nervousness, outbursts of anger, paresthesia, seizure, sleep apnea, suicidal ideation.
Dermatologic
Acne vulgaris (5%), hyperhidrosis (1%), alopecia, contact dermatitis, diaphoresis, erythema, folliculitis, hair discoloration, pruritus, seborrhea, skin rash, xeroderma.
Endocrine & Metabolic
Increased plasma estradiol concentration (3%), weight gain (1%), gynecomastia (≤1%), hot flash (≤1%), change in libido, decreased gonadotropin, fluid retention, hirsutism (increase in pubic hair growth), hypercalcemia, hyperchloremia, hypercholesterolemia, hyperglycemia, hyperkalemia, hyperlipidemia, hypernatremia, hypoglycemia, hypokalemia, inorganic phosphate retention, menstrual disease (including amenorrhea).
Gastrointestinal
Diarrhea (≥3%), gastroesophageal reflux disease, gastrointestinal hemorrhage, gastrointestinal irritation, increased appetite, nausea, vomiting.
Genitourinary
Prostate-specific antigen increase (5% to 11%), prostatitis (≥3%), ejaculatory disorder (1%), prostate induration (1%), spontaneous erections (≤1%), benign prostatic hypertrophy, difficulty in micturition, hematuria, impotence, irritable bladder, mastalgia, oligospermia, priapism, testicular atrophy, urinary tract infection, virilization.
Hepatic
Abnormal hepatic function tests, cholestatic hepatitis, cholestatic jaundice, hepatic insufficiency, hepatic necrosis, hepatocellular neoplasms, increased serum bilirubin, peliosis hepatis.
Hematologic & Oncologic
Increased hematocrit (1% to 3%), increased hemoglobin (2%), malignant neoplasm of the prostate (1%), anemia, clotting factors suppression, hemorrhage, leukopenia, polycythemia, prostate carcinoma.
Hypersensitivity
Anaphylactoid reaction, hypersensitivity reaction (including pulmonary oil microembolism).
Local
Pain at injection site (5%), erythema at injection site (1%), application site reaction (gel, solution), inflammation at the injection site.
Neuromuscular & Skeletal
Arthralgia (≥3%), back pain (≥3%), abnormal bone growth (accelerated), hemarthrosis, hyperkinesia, weakness.
Ophthalmic
Increased lacrimation.
Renal
Increased serum creatinine, polyuria.
Respiratory
Bronchitis (≥3%), nasopharyngitis (≥3%), sinusitis (≥3%), upper respiratory tract infection (≥3%), dyspnea.
<1%, postmarketing, and/or case reports:
- Abdominal pain
- Abnormal erythropoiesis
- Abscess at the injection site
- Allergic dermatitis
- Anaphylactic shock
- Anaphylaxis
- Androgenetic alopecia
- Angina pectoris
- Angioedema
- Asthma
- Breast induration
- Cardiac arrest
- Cardiac failure
- Cerebral infarction
- Cerebrovascular accident
- Cerebrovascular insufficiency
- Chest pain
- Chronic obstructive pulmonary disease
- Circulatory shock
- Cognitive dysfunction
- Confusion
- Coronary artery disease
- Coronary occlusion
- Cough
- Decreased plasma testosterone
- Decreased thyroxine binding globulin
- Decreased urinary calcium excretion
- Diabetes mellitus
- Dysuria
- Electrolyte disturbance
- Epididymitis
- Erectile dysfunction
- Hearing loss (sudden)
- Hematoma at injection site
- Hepatotoxicity (idiosyncratic) (Chalasani, 2014)
- Hyperparathyroidism
- Hypersensitivity angiitis
- Hypertriglyceridemia
- Hyperventilation
- Impaired glucose tolerance
- Increased gamma-glutamyl transferase
- Increased intraocular pressure
- Increased serum ALT
- Increased serum AST
- Increased serum prolactin
- Increased serum transaminases
- Increased serum triglycerides
- Korsakoff’s psychosis (nonalcoholic)
- Migraine
- Musculoskeletal chest pain
- Musculoskeletal pain
- Myalgia
- Myocardial infarction
- Nephrolithiasis
- Nipple tenderness
- Orgasm disturbance (male)
- Osteopenia
- Osteoporosis
- Peripheral edema
- Personality disorder
- Pharyngeal edema
- Pharyngolaryngeal pain
- Prolonged partial thromboplastin time
- Prolonged prothrombin time
- Prostatic intraepithelial neoplasia
- Pulmonary embolism
- Pure red cell aplasia
- Renal colic
- Renal pain
- Respiratory distress
- Restlessness
- Reversible ischemic neurological deficit
- Rhinitis
- Sleep disorder
- Snoring
- Spermatocele
- Syncope
- Systemic lupus erythematosus
- Tachycardia
- Testicular pain
- Thrombocytopenia
- Thromboembolism
- Thrombosis
- Tinnitus
- Transient ischemic attacks
- Urinary incontinence
- Urolithiasis
- Urticaria
- Venous insufficiency
- Venous thromboembolism
- Vesicobullous rash
- Virilization (of children, following secondary exposure to topical gel [advanced bone age, aggressive behavior, enlargement of clitoris requiring surgery, enlargement of penis, increased erections, increased libido, pubic hair development])
- Vitreous detachment
- Voice disorder
Metabolism/Transport effects
Drug Interactions from Deposteron 250
Blood Glucose Lowering Agents: Androgens may enhance the hypoglycemic effect of Blood Glucose Lowering Agents. Risk C: Monitor therapy.
C1 inhibitors: Androgens may enhance the thrombogenic effect of C1 inhibitors. Risk C: Monitor therapy.
Corticosteroids (Systemic): May enhance the fluid-retaining effect of Androgens. Risk C: Monitor therapy.
CycloSPORINE (Systemic): Androgens may enhance the hepatotoxic effect of Cyclosporine (Systemic). Androgens may increase the serum concentration of Cyclosporine (Systemic). Risk D: Consider therapy modification.
Dehydroepiandrosterone: May enhance the adverse/toxic effect of Testosterone. Risk X: Avoid combination.
Vitamin K Antagonists (eg, warfarin): Androgens may enhance the anticoagulant effect of Vitamin K Antagonists. Risk D: Consider therapy modification.
Test interactions with Deposteron
Testosterone may decrease thyroxine-binding globulin, resulting in decreased total T4; free thyroid hormone levels are not changed.
Monitoring parameters with Deposteron use
Periodic liver function tests, lipid panel, hemoglobin and hematocrit (prior to therapy, at 3 to 6 months, then annually); radiologic examination of wrist and hand every 6 months (when using in prepubertal children). Withhold initial treatment with hematocrit >50% (discontinue therapy if hematocrit exceeds 54% [Bhasin, 2010]), hyperviscosity, untreated obstructive sleep apnea, or uncontrolled severe heart failure. Monitor urine and serum calcium and signs of virilization in women treated for breast cancer. Serum glucose (may be decreased by testosterone, monitor patients with diabetes). Evaluate males for response to treatment and adverse events 3 to 6 months after initiation and then annually.
Bone mineral density
Monitor after 1 to 2 years of therapy in hypogonadal men with osteoporosis or low trauma fracture (Bhasin, 2010).
PSA
In men >40 years of age with baseline PSA >0.6 ng/mL, PSA and prostate exam (prior to therapy, at 3 to 6 months, then as based on current guidelines). Withhold treatment pending urological evaluation in patients with palpable prostate nodule or induration or PSA >4 ng/mL or if PSA >3 ng/mL in men at high risk of prostate cancer (Bhasin, 2010).
Do not treat with severe untreated BPH with IPSS symptom score >19.
Serum testosterone: After initial dose titration (if applicable), monitor 3 to 6 months after initiating treatment, then annually.
Deposteron Injection:
Testosterone enanthate or cypionate: Measure midway between injections. Adjust dose or frequency if testosterone concentration is <400 ng/dL or >700 ng/dL (Bhasin, 2010).
Testosterone undecanoate: Measure just prior to each subsequent injection and adjust the dosing interval to maintain serum testosterone in mid-normal range (Bhasin, 2010).
Reference range
Total testosterone, males:
12 to 13 years: <800 ng/dL.
14 years: <1200 ng/dL.
15 to 16 years: 100 to 1200 ng/dL.
17 to 18 years: 300 to 1200 ng/dL.
19 to 40 years: 300 to 950 ng/dL.
>40 years: 240 to 950 ng/dL.
Free testosterone, males: 9 to 30 ng/dL.
Deposteron Dosage forms
Solution, Intramuscular, as cypionate:
Depo-Testosterone: 100 mg/mL (10 mL); 200 mg/mL (1 mL, 10 mL) [contains benzyl alcohol, benzyl benzoate].
Generic: 100 mg/mL (10 mL); 200 mg/mL (1 mL, 10 mL).
Anatomic Therapeutic Chemical (ATC) Classification.
⦁ G03BA03.
Mechanism of action from Deposteron 250
Principal endogenous androgen responsible for promoting the growth and development of the male sex organs and maintaining secondary sex characteristics in androgen-deficient males.
Pharmacodynamics/Kinetics
Duration (route and ester dependent): IM: Cypionate and enanthate esters have longest duration, ≤2 to 4 weeks; Undecanoate 10 weeks; Gel: 24 to 48 hours; buccal system: 2 to 4 hours after removal.
Protein binding: 98%; bound to sex hormone-binding globulin (40%) and albumin.
Metabolism: Hepatic; forms metabolites, including dihydrotestosterone (DHT) and estradiol (both active).
Half-life elimination: Variable: 10 to 100 minutes.
Excretion: Urine (90%); feces (6%).
Effects on bleeding
No information available to require special precautions.
FAQs – Deposteron Testosterone Cypionate 250mg/ml 10ml
Order Now – Safe & Fast Shipping in the USA at SteroidsOnlineUSA.com
General Information
What is Deposteron Testosterone Cypionate?
Deposteron is a testosterone cypionate injection used for hormone replacement therapy in men with low testosterone, helping to restore normal levels and improve energy, muscle mass, and mood.
How does Deposteron work?
Deposteron increases testosterone levels in men, supporting muscle growth, strength, and overall well-being by promoting male hormone balance.
Is Deposteron available for purchase in the USA?
Yes, you can easily purchase Deposteron Testosterone Cypionate 250mg/ml at SteroidsOnlineUSA.com, with secure and fast shipping across the USA.
Usage & Dosage
How do I use Deposteron Testosterone Cypionate 250mg/ml?
Deposteron is injected intramuscularly into the gluteal muscle. The typical dosage is 50mg to 400mg every 2-4 weeks, but follow your healthcare provider’s advice.
Can Deposteron help with muscle building?
Yes, Deposteron can promote muscle growth and enhance recovery by normalizing testosterone levels, making it popular among bodybuilders and athletes.
Safety & Side Effects
What are the common side effects of Deposteron?
Side effects may include acne, water retention, mood changes, and increased blood pressure. Long-term use may elevate the risk of cardiovascular events or prostate issues.
Is Deposteron safe for women?
Deposteron is not suitable for women, especially those who are pregnant or breastfeeding, due to its potential to cause virilization and other hormonal imbalances.
Shipping & Purchase
How fast is the shipping for Deposteron in the USA?
We provide fast, discreet shipping through SteroidsOnlineUSA.com. Your order typically arrives within 3-5 business days, ensuring both speed and privacy.
Medical & Health Considerations
Do I need a prescription to buy Deposteron?
Yes, Deposteron is a controlled substance, so you will need a prescription to buy it. Always use under the guidance of a licensed healthcare provider.
Can Deposteron be used by athletes?
Although athletes may use testosterone to improve performance, it’s important to consult a doctor first as improper use can lead to serious health risks and side effects.
Product & Storage
How do I store Deposteron Testosterone Cypionate?
Keep Deposteron stored at room temperature, away from light. Make sure it’s out of reach of children and pets for safety.
What does each vial of Deposteron contain?
Each 10ml vial contains 250mg/ml of Testosterone Cypionate, a long-acting testosterone ester designed for hormone replacement therapy.
Still have questions? Visit SteroidsOnlineUSA.com or place your order now for fast, secure shipping in the USA!
Buy steroids online USA.
https://medium.com/@ibrahimneffer/deposteron-testosterone-cypionate-injection-c8dc1623b8bbmedium.com
Related products
Dynabol Buy Online in USA 100mg/ml 10 ml Medical Pharma
$93.00Add to cartMetandienone (pINN); Metandienon; Metandienona; Métandiénone; Metandienoni; Metandienonum; Methandrostenolone; NSC-42722. 17β-Hydroxy-17α-methylandrosta-1,4 dien-3-one. Метандиенон.
C20H28O2 = 300.4.
CAS — 72-63-9.
ATC — A14AA03; D11AE01.
ATC Vet — QA14AA03; QD11AE01.
NOTE. The following terms have been used as ‘street names’ or slang names for various forms of Methandienone: Iron Brew.
Pharmacopoeias. In Pol
Winstrol Pills Buy 15mg/tab 100 tabs – M P
$93.00Add to cartAndrostanazol; Androstanazole; Estanazol; Estanozolol; Methylstanazole; Metistanazol; NSC-43193; Stanotsololi; Stanozololis; Stanozololum; Stanozolum; Sztanozolol; Win-14833. 17α-Methyl-2′H-5α-androst-2-eno[3,2-c]pyrazol-17β-ol.
Testosterone Online Purchase Enanthate 250mg/ml MP
$93.00Add to cartC26H40O3 = 400.6.
CAS — 315-37-7.
ATC — G03BA03.
ATC Vet — QG03BA03.USP 31(Testosterone Enanthate). A white or creamy-white crystalline powder. It is odourless or has a faint odour characteristic of heptanoic acid. Insoluble in water; very soluble in ether; soluble in vegetable oils. Store at a temperature of 2°to 8°. Protect from light.
Testosterone E contains: 250mg 250 mg / ml Testosterone Enanthate.
Dianabol (Methandrostenolone) 20mg/tab 100 tabs – Medical Pharma Steroids USA
$93.00Add to cartMetandienone (pINN); Metandienon; Metandienona; Métandiénone; Metandienoni; Metandienonum; Methandrostenolone; NSC-42722. 17β-Hydroxy-17α-methylandrosta-1,4 dien-3-one.
Pay with WISE APP or Remitly
Pay with WISE App or Remitly
Fast money transfers from USA for fast delivery of steroids
Secure delivery in USA
100% reliable shipping in USA
24x7 Support
Online 24 hours
Low cost delivery
Great shipping prices in USA
BULK ORDER DISCOUNT
If you are a reseller in the USA you can get a special DISCOUNT, we can give you up to 50% or more on bulk orders. If you want to make a bulk order, we can negociate for orders of over USD$4,000, contact us by email.
Steroids info