Category
- Best Peptides for muscle growth
- Geno Pharma Domestic Warehouse 2 (Canada&USA)
- GPH(Domestic Shipping US) Warehouse 1
- Human Pharma Premium
- Phar Labs Premium-Select
- Steroids on Sale USA, Real Steroids Online
- New arrivals in USA
- Most popular steroids in USA
- Antiestrogens / Gonadotropins
- Bangkok Steroid USA
- Biopharma Steroid USA
- British Dragon
- Anabolic Steroids for Horses
- Fat-burners
- Gen Pharma USA
- Medical Pharma Steroid USA
- Medical Tech Steroid USA
- Novocrine Steroids
- HGH USA
- Omega Labs Steroid USA
- Rotterdam Steroids USA
- SARMs USA
- Sciroxx
- Sydgroup Steroid USA
- Big vetenary Steroid USA
- Watson Steroids
- XT Labs Steroids
Most Popular steroids USA
-
 Primobolan for sale 100mg 10 ml Human Pharma in USA
$115.00
Primobolan for sale 100mg 10 ml Human Pharma in USA
$115.00 -
 NPP Steroid 100mg 10 ml Premium Domestic USA
NPP Steroid 100mg 10 ml Premium Domestic USA
$99.00Original price was: $99.00.$80.00Current price is: $80.00. -
 Anavar for Sale in USA – 10mg 80 Tabs GPH-Premium
Anavar for Sale in USA – 10mg 80 Tabs GPH-Premium
$110.00Original price was: $110.00.$80.00Current price is: $80.00. -
 Buy Anavar 10mg – Purchase Geno Pharma
$99.00
Buy Anavar 10mg – Purchase Geno Pharma
$99.00 -
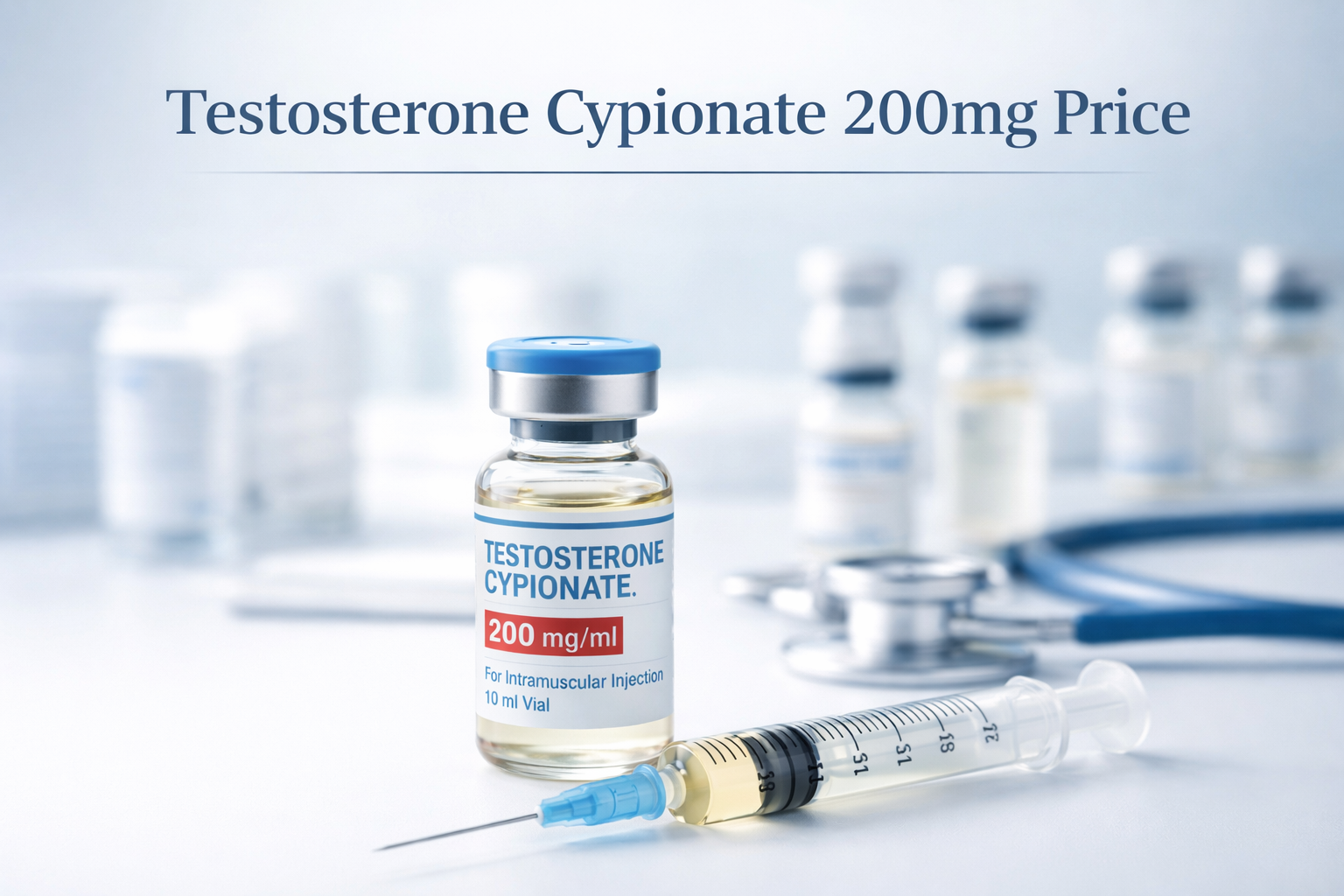
 Testosterone Cypionate Buy 300mg 10ml Geno Pharma
$99.00
Testosterone Cypionate Buy 300mg 10ml Geno Pharma
$99.00 -
 Buy Testosterone E 300mg 10 ml Geno Pharma Domestic USA/CA
$99.00
Buy Testosterone E 300mg 10 ml Geno Pharma Domestic USA/CA
$99.00 -
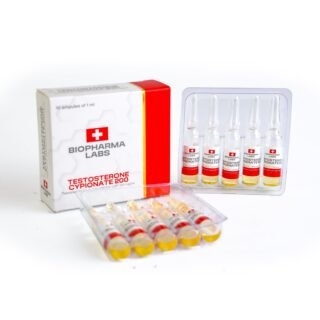
 Testosterone Cypionate 200 Biopharma 10 amp
Testosterone Cypionate 200 Biopharma 10 amp
$99.00Original price was: $99.00.$72.00Current price is: $72.00. -
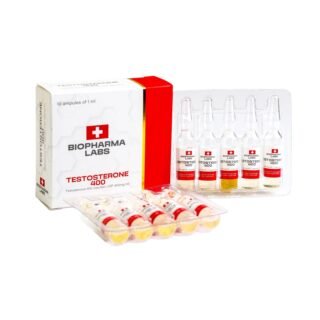
 Testosterone 400 Biopharma 10 Ampoules
Testosterone 400 Biopharma 10 Ampoules
$99.00Original price was: $99.00.$75.00Current price is: $75.00. -
 Eq 300 steroid Rotterdam 10ml
Eq 300 steroid Rotterdam 10ml
$79.00Original price was: $79.00.$69.00Current price is: $69.00. -
 Steroid Deca Geno Pharma 300mg 10ml
Steroid Deca Geno Pharma 300mg 10ml
$110.00Original price was: $110.00.$99.00Current price is: $99.00. -
 Boldenone Cypionate 200 mg / 10 mL Geno Pharma
Boldenone Cypionate 200 mg / 10 mL Geno Pharma
$90.00Original price was: $90.00.$85.00Current price is: $85.00. -
 Somatrox XT Labs 150 IU x 10 vials (15 ui each)
Somatrox XT Labs 150 IU x 10 vials (15 ui each)
$450.00Original price was: $450.00.$350.00Current price is: $350.00.

Provir (Mesterolone) 50mg/tab 100 tabs – Medical Pharma
$93.00
Pharmacologic Category
Androgen.
Dosing: Adult
50mg every days for duration of 4-8 weeks.
Dosing: Geriatric
Refer to adult dosing.
Dosing: Pediatric
No information in pediatric population available.
Dosing: Renal impairment
No dosage adjustment provided in manufacturer’s labeling; use with caution due to propensity to cause edema.
Dosing: Hepatic impairment
No dosage adjustment provided in manufacturer’s labeling; use with caution.
Share this page:
- Share on X (Opens in new window) X
- Share on Facebook (Opens in new window) Facebook
- Email a link to a friend (Opens in new window) Email
- Share on LinkedIn (Opens in new window) LinkedIn
- Share on Reddit (Opens in new window) Reddit
- Share on Pinterest (Opens in new window) Pinterest
- Share on Telegram (Opens in new window) Telegram
- Share on WhatsApp (Opens in new window) WhatsApp
- Share on Tumblr (Opens in new window) Tumblr
Provir (Mesterolone) 50mg/tab 100 tabs – Medical Pharma Steroid in USA
Provir (Mesterolone) Use: Labeled indications
Has been used in the treatment of advanced malignant neoplasms of the breast in postmenopausal.
Storage/Stability
Keep at room temperature 15-30°C (59-86°F). Do not freeze. This drug should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Warming and shaking the vial should re-dissolve any crystals that may have formed during storage at temperatures lower than recommended. Protect from sun light
Contraindications
Hypersensitivity to Mesterolone or any component of the formulation.
Men with carcinomas of the breast or with known or suspected carcinomas of the prostate; women who are or may become pregnant.
Documentation of allergenic cross-reactivity for androgens is limited. However, because of similarities in chemical structure and/or pharmacologic actions, the possibility of cross-sensitivity cannot be ruled out with certainty.
Warnings/Precautions
• Gynecomastia: May cause Gynecomastia.
• Hepatic effects: Prolonged use and/or high doses may cause peliosis hepatis or liver cell tumors, which may not be apparent until liver failure or intra-abdominal hemorrhage develops. Discontinue in case of cholestatic hepatitis with jaundice or abnormal liver function tests.
• Venous thromboembolism: Venous thromboembolic events, including deep vein thrombosis (DVT) and pulmonary embolism (PE), have been reported with testosterone products. Evaluate patients with symptoms of pain, edema, warmth, and erythema in the lower extremity for DVT and those with acute shortness of breath for PE. Discontinue therapy if a venous thromboembolism is suspected.
Disease related concerns:
• Breast cancer: Use with caution in patients with breast cancer; may cause hypercalcemia by stimulating Osteolysis.
• Edematous conditions: Use with caution in patients with conditions influenced by edema (eg, cardiovascular disease, migraine, seizure disorder, renal impairment); may cause fluid retention.
• Hepatic impairment: Use with caution in patients with hepatic impairment.
Pregnancy risk factor
X
Category X: Studies in animals or human beings have demonstrated fetal abnormalities, or there is evidence of fetal risk based on human experience, or both, and the risk of the use of the drug in pregnant women clearly outweighs any possible benefit. The drug is contraindicated in women who are or may become pregnant.
Pregnancy considerations
Use is contraindicated in women who are or may become pregnant; masculinization of the fetus has been reported.
Lactation
Excretion in breast milk unknown/not recommended
Breast feeding considerations
It is not known if drostanolone is excreted in breast milk. Due to the potential for serious adverse reactions in the nursing infant, breast feeding is not recommended.
Adverse reactions
Frequency unknown.
Cardiovascular: Edema.
Central nervous system: Depression, excitation, insomnia.
Dermatologic: Acne (females and prepubertal males).
Also reported in females: Hirsutism, male-pattern baldness.
Endocrine & metabolic: Electrolyte imbalances, glucose intolerance, gonadotropin secretion inhibited, gynecomastia, HDL decreased, LDL increased, libido changes.
Also reported in females: Clitoral enlargement, menstrual irregularities
Genitourinary:
Prepubertal males: Increased or persistent erections, penile enlargement.
Postpubertal males: Bladder irritation, epididymitis, impotence, oligospermia, priapism (chronic), testicular atrophy, testicular function inhibited.
Hematologic: Prothrombin time increased, suppression of clotting factors.
Hepatic: Alkaline phosphatase increased, ALT increased, AST increased, bilirubin increased, cholestatic jaundice, hepatic necrosis (rare), hepatocellular neoplasms, peliosis hepatis (with long-term therapy).
Neuromuscular & skeletal: CPK increased, premature closure of epiphyses (in children).
Renal: Creatinine excretion increased.
Metabolism/Transport effects
None known.
Drug interactions
Corticosteroids, Warfarin and Antidiabetic agents.
Test interactions
May suppress factors II, V, VII, and X; may increase PT; may decrease thyroxine-binding globulin and radioactive iodine uptake
Monitoring parameters
Liver function tests, cholesterol profile, hemoglobin/hematocrit; INR/PT in patients on anticoagulant therapy.
Children: Radiographs of left wrist and hand every 6 months (to assess bone maturation).
Adult females: Signs of virilization (deepening voice, hirsutism, acne, clitoromegaly); urine and serum calcium in women with breast cancer.
Dosage forms
10 mg and 20 mg pills of steroid
Anatomic Therapeutic Chemical (ATC) Classification
⦁ A14 (no specific code ever assigned).
Mechanism of Action
Synthetic derivative of dihydrotestosterone, producing an anabolic effect and promoting protein synthesis as well as creating positive nitrogen balance in humans. Does not aromatize to estrogens.
Pharmacodynamics/Kinetics
Metabolism: Hepatic
Elimination: Kidney
Half-life: 2-3 days
Local anesthetic/Vasoconstrictor precautions
No information available to require special precautions.
Effects on dental treatment
No significant effects or complications reported.
Effects on bleeding
No information available to require special precautions.
Buy anabolic steroids online USA.
Related products
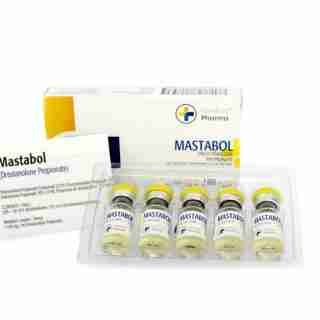
Mastabol 100mg/ml 10ml – Medical Pharma Buy in USA
$93.00Add to cartMastabol 100mg/ml (10ml) by Medical Pharma is a premium Drostanolone Propionate formula designed to enhance muscle hardness, strength, and fat loss. Ideal for cutting cycles, it promotes a lean, defined physique while maintaining high performance. Available for secure purchase in the USA with fast shipping and a trusted buying process.
Equipoise 200mg/ml 10 ml – Medical Pharma
$93.00Add to cartBa-29038; Boldenone Undecylenate (USAN); Boldenone, Undecylenate de; Boldenoni Undecylenas; 1-Dehydrotestosterone (boldenone); Undecilenato de boldenona. 17β-Hydroxyandrosta-1,4-dien-3-one 17-(undec-10-enoate). Болденона Ундециленат.
C30H44O3 = 452.7.
CAS — 846-48-0 (boldenone); 13103-34-9 (boldenone undecenoate).
Equipoise contains: 200mg.
200 mg / ml Boldenone Undecylenate.Pharmacologic Category: Androgen.
Dosing
a) 1 mg/kg IM; may repeat in 1 week intervals (most will respond with one or two treatments).
b) 3 mg/kg IM; repeated at 1 week intervals (Robinson 1987).Anavar For Sale Online 15mg/100tabs MP | Purchasing Anavar US
$99.00Add to cartAnavar (Oxandrolone) 15mg 100 Tablets – Medical Pharma
Anavar (Oxandrolone) 15mg by Medical Pharma is a high-quality anabolic steroid known for its muscle-preserving and performance-enhancing properties. With 100 tablets per bottle, each containing 15mg of Oxandrolone (NSC-67068, SC-11585), it supports lean muscle retention, fat loss, and recovery while minimizing water retention. Its mild androgenic profile makes it a preferred choice for both men and women looking for effective yet controlled performance enhancement.
Testesterone P 100mg/ml 10ml (Testosterone Propionate) – Medical Pharma
$93.00Add to cartC22H32O3 = 344.5.
CAS — 57-85-2.
ATC — G03BA03.
ATC Vet — QG03BA03.SP 31(Testosterone Propionate). White or creamy-white, odourless, crystals or crystalline powder. Insoluble in water; freely soluble in alcohol, in dioxan, in ether, and in other organic solvents; soluble in vegetable oils. Protect from light.
Testosterone contains: 100mg 100 mg / ml Testosterone Propionate.
Pay with WISE APP or Remitly
Pay with WISE App or Remitly
Fast money transfers from USA for fast delivery of steroids
Secure delivery in USA
100% reliable shipping in USA
24x7 Support
Online 24 hours
Low cost delivery
Great shipping prices in USA
BULK ORDER DISCOUNT
If you are a reseller in the USA you can get a special DISCOUNT, we can give you up to 50% or more on bulk orders. If you want to make a bulk order, we can negociate for orders of over USD$4,000, contact us by email.
Steroids info